With increasing scrutiny on consumer safety of cannabis products coupled...
Read More


Good Manufacturing Practices (GMP) play a pivotal role in industries that demand consistency, safety, and quality—such as the pharmaceutical, food, dietary supplements, and now, the cannabis industry. With increasing scrutiny on consumer safety of cannabis products coupled with the impending rescheduling of marijuana to Schedule III of the Controlled Substances Act, GMP compliance is essential to all cannabis processing operations. This article explores what GMP compliance means in the context of cannabis extraction equipment and why it’s critical for the future of the industry.
Good Manufacturing Practices (GMP) are a system of regulations enforced by government agencies like the FDA (U.S. Food and Drug Administration) or EMA (European Medicines Agency) to ensure that products are consistently produced and controlled according to strict quality standards. GMP regulations cover all aspects of production, from raw materials and equipment to employee hygiene and training.
For extraction equipment, GMP compliance means the machinery is designed, built, and documented to ensure that it meets the operational requirements for producing high-quality cannabis extracts. This includes standards for:
The Importance of GMP Compliance in Cannabis Extraction
The cannabis industry is rapidly evolving, particularly in regions where medical cannabis is legal or where adult-use markets are becoming more regulated. As cannabis moves closer to mainstream food, dietary supplements, and pharmaceutical production, GMP compliance will no longer be optional but a requirement for companies to be compliant and competitive.
GMP-compliant extraction equipment ensures that cannabis extracts meet the highest standards of quality, safety, and consistency—which is essential as governments and consumers alike become more focused on consumer safety. GMP compliance not only helps ensure that products are free from contaminants like pesticides, heavy metals, or microbial impurities, but it also ensures that extraction processes are consistent across batches.
Key benefits of using GMP-compliant equipment in cannabis extraction include:
Key Components of GMP-Compliant Extraction Equipment
To meet GMP standards, cannabis extraction equipment must meet several important criteria. These criteria help ensure that the equipment is safe, efficient, and reliable in producing high-quality cannabis extracts for various uses.
Preparing for the Future of Cannabis Extraction
As cannabis is poised for potential rescheduling in the U.S., with the possibility of it moving to Schedule III under the Controlled Substances Act, GMP compliance will be more important than ever. The removal of Schedule I restrictions would allow for more extensive research and development of cannabis-derived drugs, creating opportunities for pharmaceutical companies and research institutions to collaborate with existing extraction labs. Schedule III will also create an instant and unprecedented opportunity to market pharmaceutical products containing THC and CBD via compounding pharmacies in the United States.
For extraction companies, now is the time to invest in GMP-compliant equipment. This will not only future-proof operations for potential regulatory changes but also allow for entry into new markets where cannabis extracts are used for pharmaceutical applications. By adhering to GMP guidelines, cannabis extraction companies can position themselves as leaders in a rapidly growing and increasingly regulated industry.
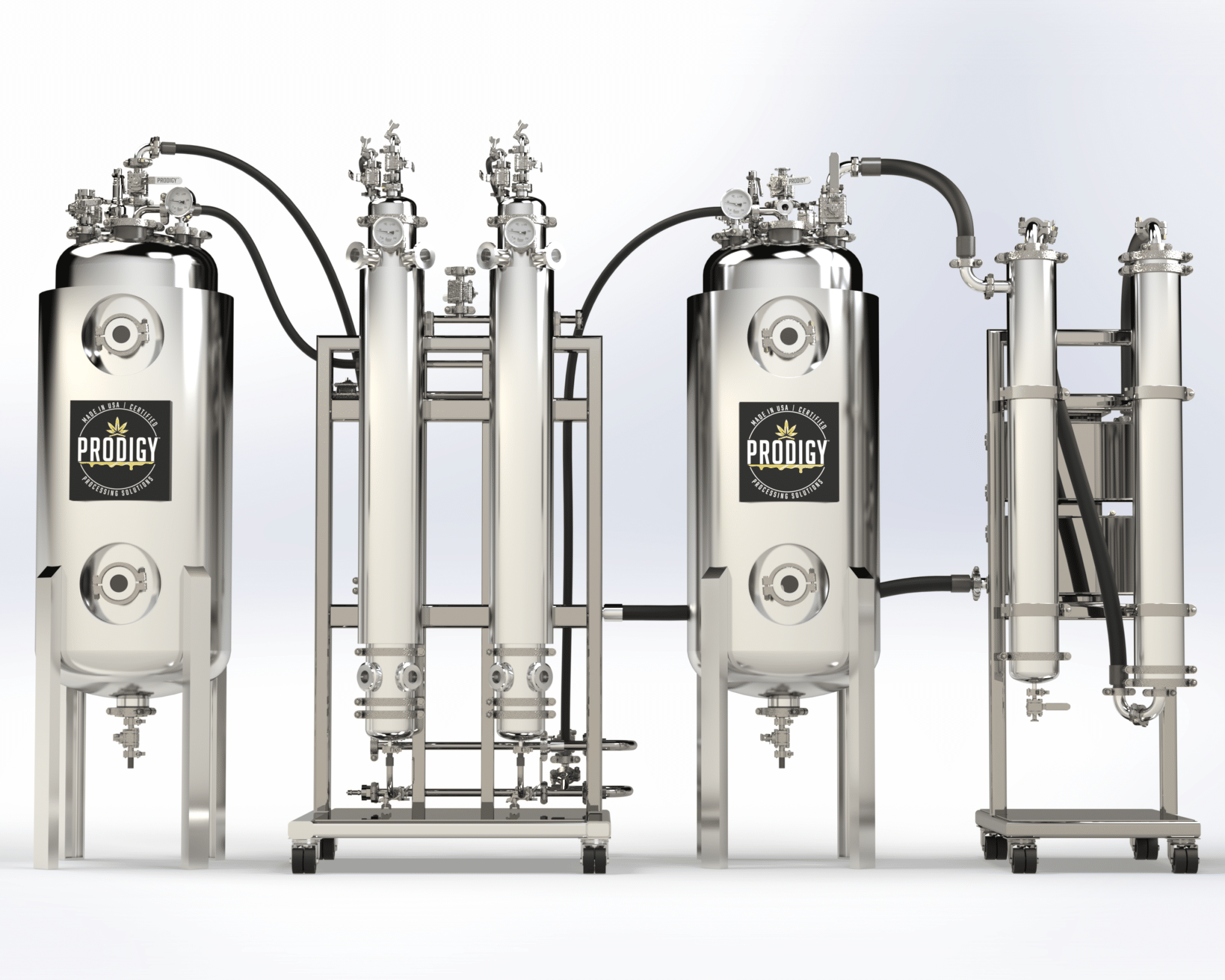
Crude Oil / Precursor Extract | Chemical Isolation, Separation, and Purification | Filtration / HTE (Terpene) Separation | Wiped Film Distillation | C1D1 and C1D2 Rooms | Clean Rooms | Freeze Dryers | Analytical Testing | Equipment Installation and Training | Turnkey Extraction Solutions from Plant to 99%+ Cannabinoid API
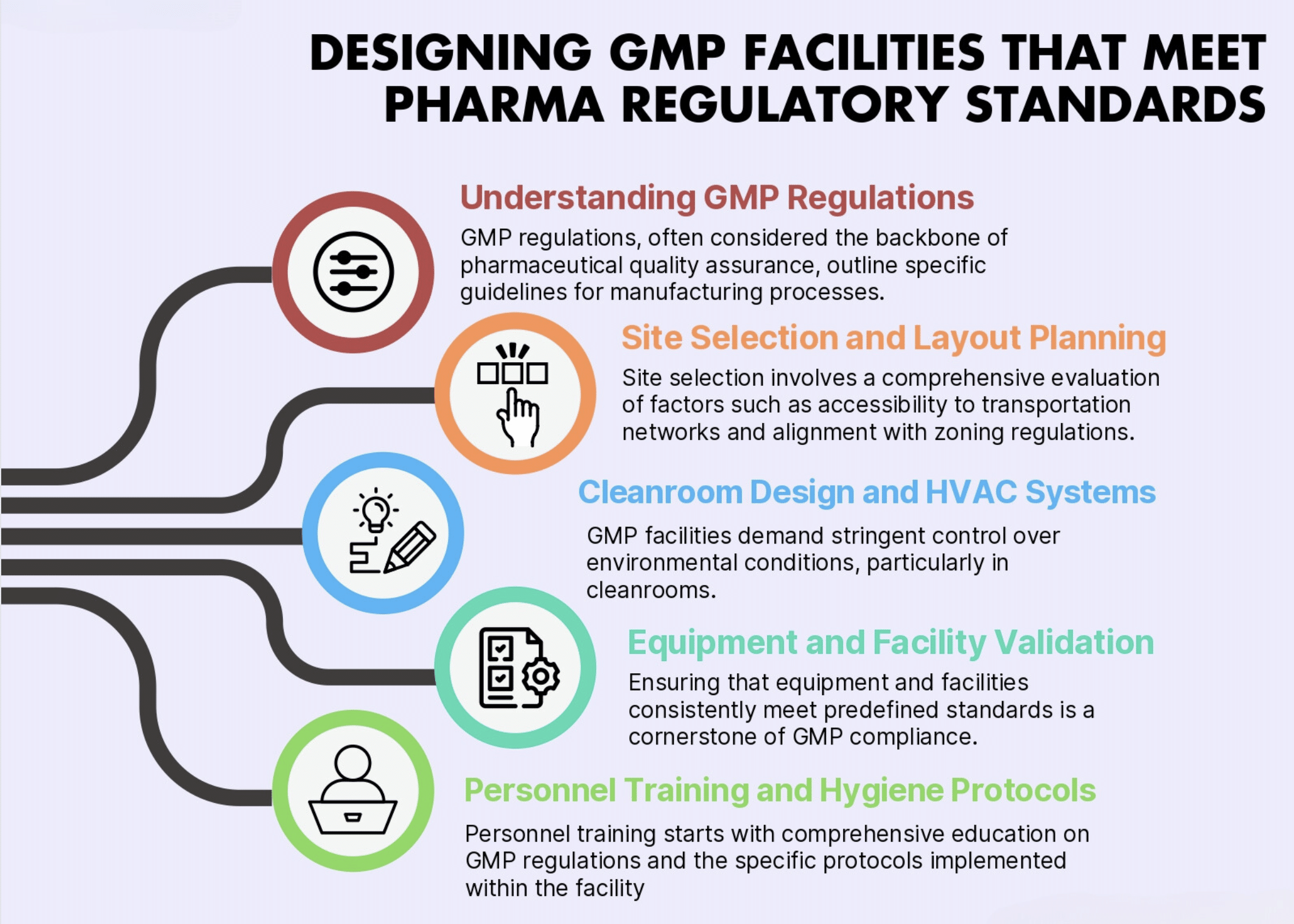
Full Design-Build Services for Cannabinoid Pharmaceutical Manufacturing Full Facility Design | GMP Compliant Lab Design | FDA cGMP | EU GMP | Worldwide GMP | Architectural Drawings | MEP Plans | Construction Documents | Construction Services

Basic Crude Extraction (cannabinoid precursors) | Distillate Production | Isolate Production | Cannabinoid APIs | Delta-9 THC (plant-based Dronabinol i.e., tetrahydrocannabinol) | CBD (cannabidiol) | CBDV (cannabidivarin) | THCV (tetrahydrocannabivarin) | CBN (cannabinol) | CBG (cannbigerol) | And more cannabinoid APIs of 99%+ purity

Legal & Regulatory | GMP Compliance | Standard Operating Procedures | Food, Dietary Supplements, and Pharmaceutical SOPs | Safety Protocols | Business / Revenue Opportunities | Maximizing Optimization, ROI and Profitability
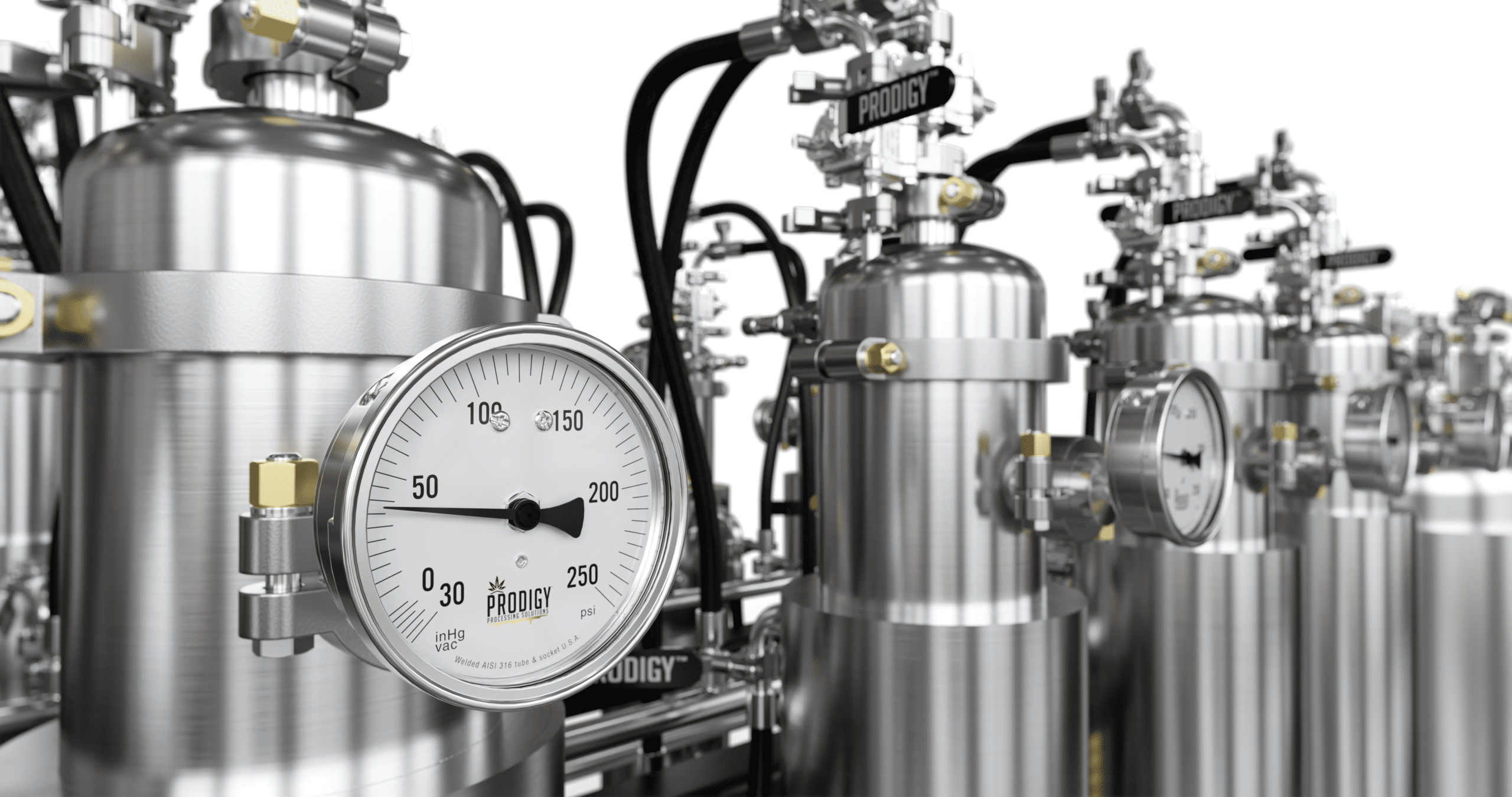
World Class GMP Compliant Cannabis Extraction Technology

The Professional's Choice for Cannabis Extraction

With increasing scrutiny on consumer safety of cannabis products coupled...
Read MoreWith increasing scrutiny on consumer safety of cannabis products coupled...
Read MoreWith increasing scrutiny on consumer safety of cannabis products coupled...
Read More