With increasing scrutiny on consumer safety of cannabis products coupled...
Read More


With the anticipated rescheduling of cannabis to Schedule III by the DEA, state-licensed cannabis extraction and processing labs have an incredible ROI opportunity to capitalize upon by pivoting into pharmaceutical cannabinoid API manufacturing. Here are the key definitions, reasons and benefits for making this strategic transition:
The active ingredient in a pharmaceutical drug is called an active pharmaceutical ingredient (API). An example of an API is the acetaminophen contained in a pain relief tablet such as Tylenol. Active ingredients are the substances in drugs that are responsible for the beneficial health effects experienced by consumers. Generally an API is of at least 99% purity.
Upon DEA rescheduling of marijuana to Schedule III, Delta-9 THC (tetrahydrocannabinol) will be a highly desired API for pharmaceutical drug products in the USA and worldwide.
Other cannabis-derived APIs, such as Cannabidiol (CBD) also constitute an incredible ROI opportunity with CBD API selling in Europe for 5,900 to 9,000 EUROS per kilogram. (Source: https://www.statista.com/statistics/1375642/europe-price-of-cbd-api-per-kg/). Due to its current scarcity, Tetrahydrocannabinol THC API is anticipated to be much more expensive, and therefore immensely lucrative to pharmaceutical API manufacturers.
In the United States, both THC and CBD have been approved by the FDA for certain pharmaceutical applications. THC (dronabinol) is the active ingredient in the approved drug products, Marinol capsules (and generics) and Syndros oral solution. CBD is the active ingredient in the approved drug product, Epidiolex. Neither THC nor CBD are approved by the FDA as food or dietary supplement ingredients, but under Schedule III they are FDA approved as active pharmaceutical ingredients.
Converting to pharmaceutical API manufacturing–including as a CMO, CDMO, or CRO–opens access to the broader pharmaceutical market throughout the United States, Europe, Australia, and worldwide, which is significantly larger and more lucrative than the state-confined recreational or medical cannabis markets. This includes opportunities to supply APIs to pharmaceutical companies globally, thereby expanding market reach and revenue potential.
As of Aug 2024: Cost of 1 lb of cannabis trim containing approx. 10% THCA/THC by volume by weight is $10 – $15 (confirmed for California and Oklahoma). Delta-9 THC API market rates in EU pharmaceutical industry range $20,000 – $45,000 while CBD API is $5,000 – $9,000 (estimates do your own research).

Building Trust with Consumers and Partners:
Differentiation from Competitors:
Advanced Technology and Processes:
Improved Facility Utilization:
Enhanced Regulatory Compliance:
Preemptive Compliance with Future Regulations:
Adaptability to Market Changes:
Potential for Future Licensing and Expansion:
Converting a state-licensed cannabis extraction and processing lab to pharmaceutical API manufacturing offers numerous strategic advantages. From enhanced regulatory compliance and market expansion to increased credibility and operational efficiency, this transition positions the lab for long-term success and growth. As the DEA moves towards rescheduling cannabis to Schedule III, now is the opportune time to make this strategic pivot and capitalize on expanding and highly lucrative opportunities within the domestic and international pharmaceutical industry.
For further reading and sources:
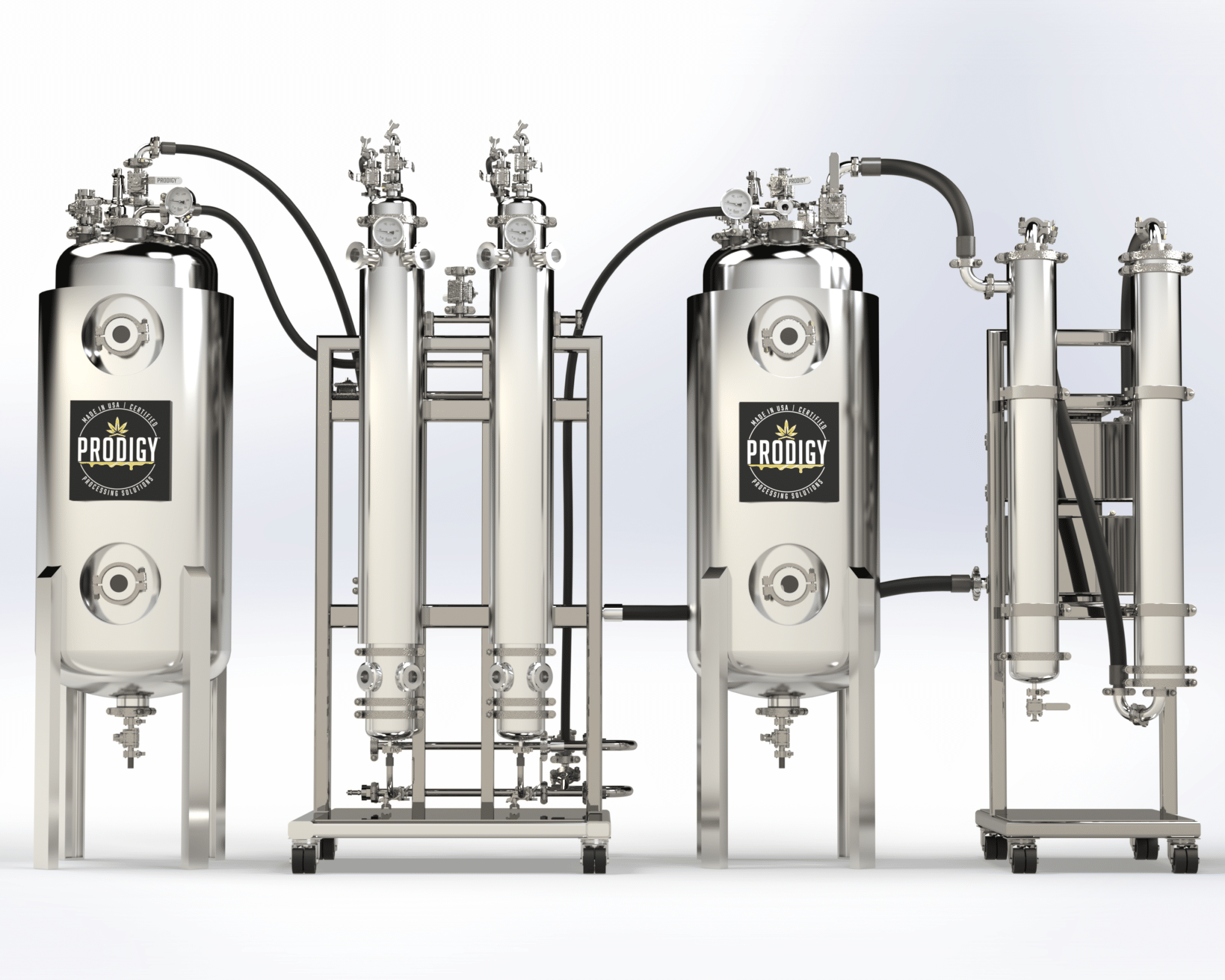
Crude Oil / Precursor Extract | Chemical Isolation, Separation, and Purification | Filtration / HTE (Terpene) Separation | Wiped Film Distillation | C1D1 and C1D2 Rooms | Clean Rooms | Freeze Dryers | Analytical Testing | Equipment Installation and Training | Turnkey Cannabinoid API Equipment Solutions from Plant to 99%+ Cannabinoid API
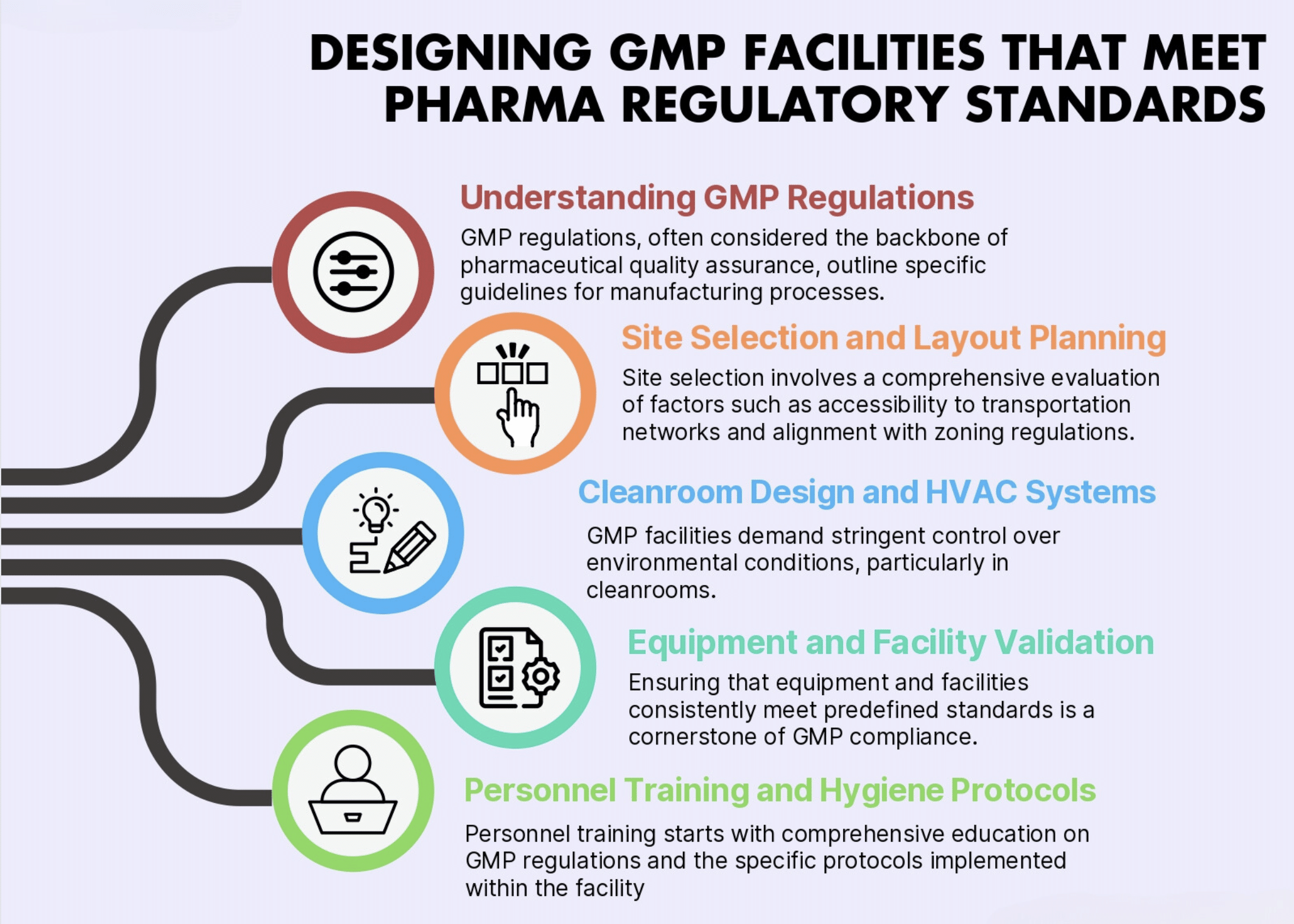
Full Design-Build Services for Cannabinoid Pharmaceutical Manufacturing Full Facility Design | GMP Compliant Lab Design | FDA cGMP | EU GMP | Worldwide GMP | Architectural Drawings | MEP Plans | Construction Documents | Construction Services

Basic Crude Extraction (cannabinoid precursors) | Distillate Production | Isolate Production | Cannabinoid APIs | Delta-9 THC (plant-based Dronabinol i.e., tetrahydrocannabinol) | CBD (cannabidiol) | CBDV (cannabidivarin) | THCV (tetrahydrocannabivarin) | CBN (cannabinol) | CBG (cannbigerol) | And more cannabinoid APIs of 99%+ purity

Legal & Regulatory | GMP Compliance | Standard Operating Procedures | Food, Dietary Supplements, and Pharmaceutical SOPs | Safety Protocols | Business / Revenue Opportunities | Maximizing Optimization, ROI and Profitability
World Class cGMP and GMP Cannabis Extraction Technology

The Professional's Choice for Cannabis Extraction Technology

With increasing scrutiny on consumer safety of cannabis products coupled...
Read MoreWith increasing scrutiny on consumer safety of cannabis products coupled...
Read MoreWith increasing scrutiny on consumer safety of cannabis products coupled...
Read MoreThe equipment you choose for your cannabis extraction business is...
Read MoreDelta-9 THC API Calculator | Input Your Own Values The...
Read MoreApplying a cost-benefit analysis to your capital expenditures In the...
Read MoreTaking the mystery out of Good Manufacturing Practices Forward Note:...
Read MoreSupport of the DEA’s proposed rescheduling of marijuana from Schedule...
Read MoreWhat processors and dispensary owners need to know By Marc...
Read More