Cannabinoid API Manufacturing Benefits and Calculator
Delta-9 THC API Calculator | Input Your Own Values

The Profit Opportunity is Immense
With the anticipated rescheduling of cannabis to Schedule III by the DEA, state-licensed cannabis extraction and processing labs have an incredible ROI opportunity to capitalize upon by pivoting into pharmaceutical cannabinoid API manufacturing. Here are the key definitions, reasons and benefits for making this strategic transition:
What is an API?
The active ingredient in a pharmaceutical drug is called an active pharmaceutical ingredient (API). An example of an API is the acetaminophen contained in a pain relief tablet such as Tylenol. Active ingredients are the substances in drugs that are responsible for the beneficial health effects experienced by consumers. Generally an API is of at least 99% purity.
What do APIs have to do with cannabis extraction?
Upon DEA rescheduling of marijuana to Schedule III, Delta-9 THC (tetrahydrocannabinol) will be a highly desired API for pharmaceutical drug products in the USA and worldwide.
Other cannabis-derived APIs, such as Cannabidiol (CBD) also constitute an incredible ROI opportunity with CBD API selling in Europe for 5,900 to 9,000 EUROS per kilogram. (Source: https://www.statista.com/statistics/1375642/europe-price-of-cbd-api-per-kg/). Due to its current scarcity, Tetrahydrocannabinol THC API is anticipated to be much more expensive, and therefore immensely lucrative to pharmaceutical API manufacturers.
In the United States, both THC and CBD have been approved by the FDA for certain pharmaceutical applications. THC (dronabinol) is the active ingredient in the approved drug products, Marinol capsules (and generics) and Syndros oral solution. CBD is the active ingredient in the approved drug product, Epidiolex. Neither THC nor CBD are approved by the FDA as food or dietary supplement ingredients, but under Schedule III they are FDA approved as active pharmaceutical ingredients.
Converting to pharmaceutical API manufacturing–including as a CMO, CDMO, or CRO–opens access to the broader pharmaceutical market throughout the United States, Europe, Australia, and worldwide, which is significantly larger and more lucrative than the state-confined recreational or medical cannabis markets. This includes opportunities to supply APIs to pharmaceutical companies globally, thereby expanding market reach and revenue potential.
That's the fancy pharmaceutical name for Delta-9 THC, the main psychoactive component of the cannabis plant, which may be manufactured synthetically or derived from botanical cannabis.
State-legal cannabis extraction labs in the United States have been extracting THC and other cannabinoids from botanical cannabis for over a decade.
TAKEAWAY: A Cannabinoid API is born!!
*Prodigy has turnkey life science lab equipment solutions to isolate the CBD and THC API at 99%+ purity. Our team of extraction and compliance experts have been advising and equipping cannabis extraction labs for their transition to pharmaceutical API manufacturing via GMP compliance, state licensing, and DEA and FDA registration to capitalize on this momentous opportunity.
Cannabinoid API Calculator
Crude Cannabis Oil to C₂₁H₃₀O₂ Δ-9 Tetrahydrocannabinol (THC)
As of Aug 2024: Cost of 1 lb of cannabis trim containing approx. 10% THCA/THC by volume by weight is $10 – $15 (confirmed for California and Oklahoma). Delta-9 THC API market rates in EU pharmaceutical industry range $20,000 – $45,000 while CBD API is $5,000 – $9,000 (estimates do your own research).
Steps to Become an FDA Compliant, State Licensed, and DEA Registered Pharmaceutical Manufacturing Facility

Let's GET STARTED
Transition your lab to FDA cGMP
pharmaceutical compliance and DEA registration!
More Reasons to Upgrade to Pharmacuetical Cannabinoid API Manufacturing
Enhanced Credibility and Consumer Trust
Building Trust with Consumers and Partners:
- Producing pharmaceutical-grade cannabinoid APIs enhances a lab’s credibility and reputation for quality and safety.
- This trust can attract partnerships with established pharmaceutical companies and foster long-term business relationships.
Differentiation from Competitors:
- Being an early adopter of pharmaceutical standards can differentiate a lab from competitors who may still be operating under less stringent cannabis-specific regulations.
Operational and Technological Advantages
Advanced Technology and Processes:
- Transitioning to cannabinoid API manufacturing necessitates the adoption of advanced extraction and processing technologies, improving operational efficiency and product consistency.
- These technological advancements can also streamline processes, reduce costs, and maximize ROI and profitability in the long run.
Improved Facility Utilization:
- Upgrading to meet pharmaceutical standards for cannabinoid API manufacturing can optimize facility use, ensuring that the lab operates at maximum efficiency, productivity, and profitability.
Both THC and CBD have been approved by the FDA for certain pharmaceutical applications. THC (dronabinol) is the active ingredient in the approved drug products, Marinol capsules (and generics) and Syndros oral solution. CBD is the active ingredient in the approved drug product, Epidiolex. Neither THC nor CBD are approved by the FDA as food or dietary supplement ingredients.
While the 2018 Farm Bill removed hemp (containing not more than 0.3% Delta-9 THC concentrate) from DEA regulation under the CSA (Controlled Substances Act), Hemp and CBD products remain subject to regulation under the Federal Food Drug & Cosmetic Act (FD&C Act) for drugs, foods, dietary supplements, cosmetics, and veterinary products.
TAKEAWAY: PHARMA is the FUTURE!!
Regulatory Alignment and Compliance
Enhanced Regulatory Compliance:
- Transitioning to pharmaceutical cannabinoid API manufacturing aligns with stricter regulatory standards, ensuring compliance with FDA and international guidelines, such as EudraLex Volume 4 GMP standards.
- This shift positions the lab to meet stringent quality control and safety requirements, which can lead to higher product quality and consumer trust.
Preemptive Compliance with Future Regulations:
- By adopting pharmaceutical standards early, labs can stay ahead of future regulatory changes and avoid potential compliance issues as the cannabis industry evolves.
- Pivoting to cannabinoid API manufacturing as a licensed pharma lab positions you to avoid the precarious regulatory environment confronting state-licensed cannabis operations
Strategic Positioning for Future Opportunities
Adaptability to Market Changes:
- As the cannabis industry continues to evolve, being positioned as a pharmaceutical cannabinoid API manufacturer allows for greater adaptability to changing market demands and regulatory landscapes.
- This flexibility ensures the lab can pivot and innovate as needed to stay competitive.
Potential for Future Licensing and Expansion:
- Successfully transitioning to cannabinoid API manufacturing can open doors for additional licensing opportunities and expansion into new product lines or therapeutic areas within the pharmaceutical industry.
Nabiximols (brand name Sativex in countries where it is approved) is a botanically derived oromucosal spray consisting of 2.7 mg of THC and 2.5 mg of CBD per spray. Nabiximols has been approved for the treatment of spasticity due to multiple sclerosis in the United Kingdom since June 2010.
Conclusion
Converting a state-licensed cannabis extraction and processing lab to pharmaceutical API manufacturing offers numerous strategic advantages. From enhanced regulatory compliance and market expansion to increased credibility and operational efficiency, this transition positions the lab for long-term success and growth. As the DEA moves towards rescheduling cannabis to Schedule III, now is the opportune time to make this strategic pivot and capitalize on expanding and highly lucrative opportunities within the domestic and international pharmaceutical industry.
For further reading and sources:
NEXT STEPS: PRODIGY CAN GET YOUR THERE!
Turnkey Cannabinoid API Manufacturing Facilities
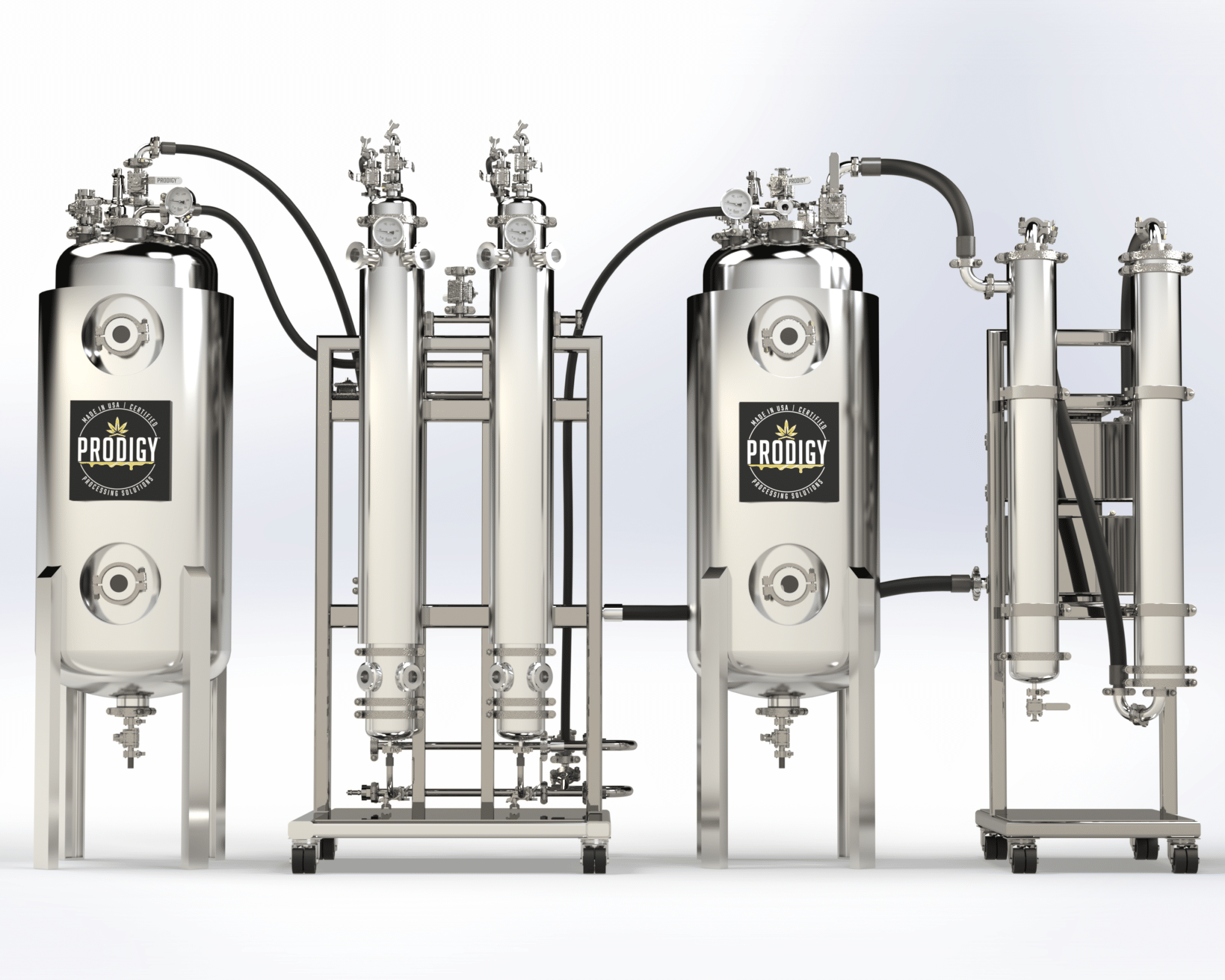
Cannabinoid API Laboratory Equipment
Crude Oil / Precursor Extract | Chemical Isolation, Separation, and Purification | Filtration / HTE (Terpene) Separation | Wiped Film Distillation | C1D1 and C1D2 Rooms | Clean Rooms | Freeze Dryers | Analytical Testing | Equipment Installation and Training | Turnkey Cannabinoid API Equipment Solutions from Plant to 99%+ Cannabinoid API
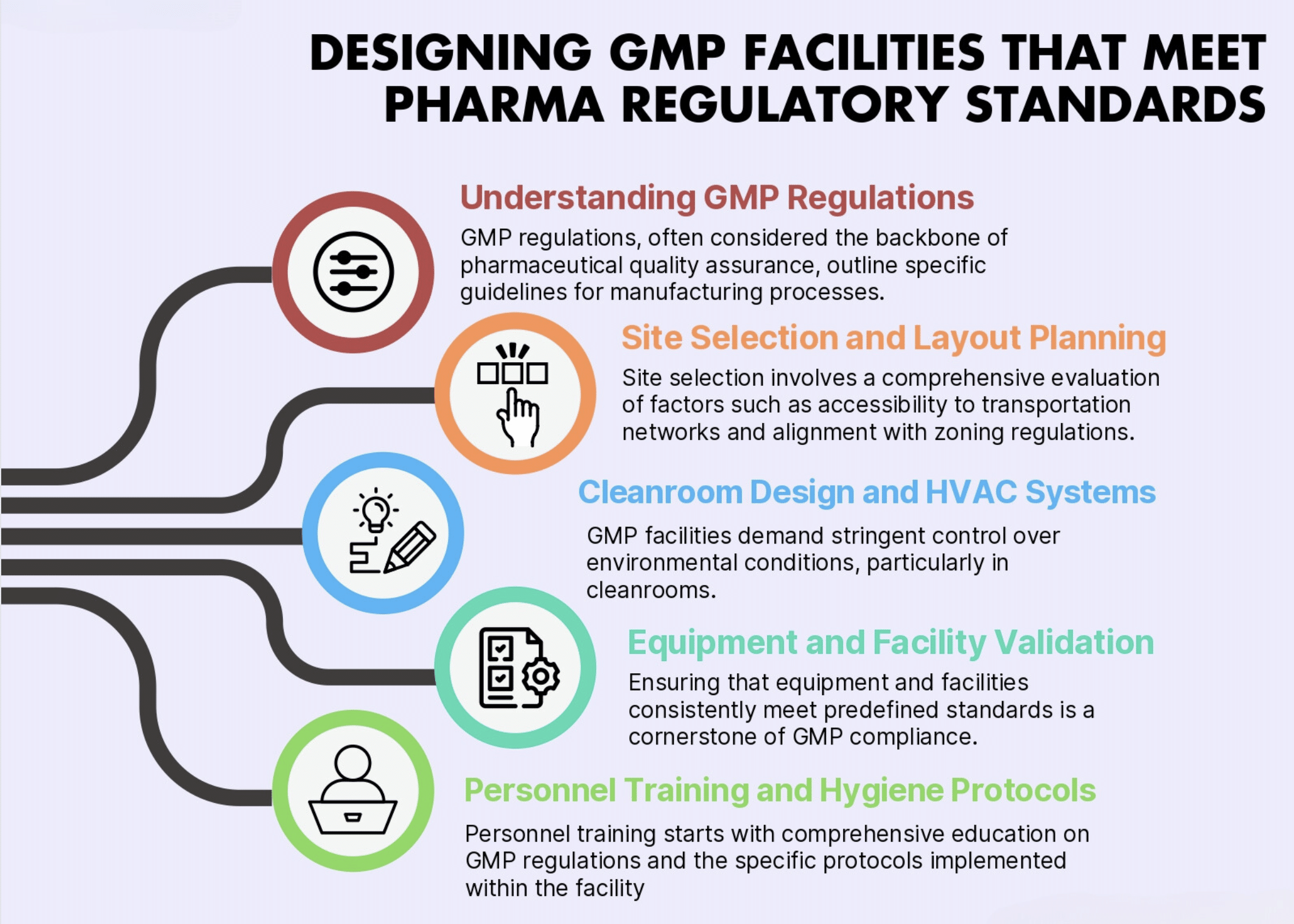
Cannabinoid API Facility and Lab Design-Build
Full Design-Build Services for Cannabinoid Pharmaceutical Manufacturing Full Facility Design | GMP Compliant Lab Design | FDA cGMP | EU GMP | Worldwide GMP | Architectural Drawings | MEP Plans | Construction Documents | Construction Services

Certified Operator Cannabinoid API Training
Basic Crude Extraction (cannabinoid precursors) | Distillate Production | Isolate Production | Cannabinoid APIs | Delta-9 THC (plant-based Dronabinol i.e., tetrahydrocannabinol) | CBD (cannabidiol) | CBDV (cannabidivarin) | THCV (tetrahydrocannabivarin) | CBN (cannabinol) | CBG (cannbigerol) | And more cannabinoid APIs of 99%+ purity

API Manufacturing Consulting & GMP SOPs
Legal & Regulatory | GMP Compliance | Standard Operating Procedures | Food, Dietary Supplements, and Pharmaceutical SOPs | Safety Protocols | Business / Revenue Opportunities | Maximizing Optimization, ROI and Profitability
Let's GET STARTED
We look forward to transitioning your cannabis lab to FDA pharmaceutical compliant extraction standards!
It is important to note that, to date, the FDA has not approved an NDA (New Drug Application) for a drug product containing botanical marijuana. However, two drug products containing synthetic Δ9-THC (as dronabinol, which is specifically the (-)-trans-Δ9-THC stereoisomer), have received FDA approval: Marinol and Syndros. Dronabinol is (at the time of this writing) a Schedule I substance under the CSA (Controlled Substances Act) unless it is contained in an FDA-approved drug product.
Marinol (dronabinol) capsules, 2.5, 5, and 10 mg, received FDA approval in 1985 for the treatment of nausea and vomiting associated with cancer chemotherapy in patients who failed to respond adequately to conventional anti-emetic treatments. In 1992, FDA approved an additional indication for the treatment of anorexia associated with weight loss in patients with acquired immunodeficiency syndrome (AIDS). Following the 1985 Marinol approval, DEA conducted a product-specific rescheduling in 1986 for “synthetic dronabinol in sesame oil and encapsulated in soft gelatin capsules,” moving it from Schedule I into Schedule II. In 1999, DEA rescheduled “synthetic dronabinol in sesame oil and encapsulated in soft gelatin capsules” again, from Schedule II into Schedule III, based on low numbers of reports of abuse of Marinol relative to marijuana.
Syndros (dronabinol) oral solution 5 mg/ml received FDA approval in 2016 for the same indications as those approved for Marinol: nausea and vomiting associated with cancer chemotherapy in patients who have failed to respond adequately to conventional antiemetic treatments and anorexia associated with weight loss in patients with AIDS. Following FDA approval, DEA conducted a product-specific rescheduling in 2017 for “FDA-approved products containing dronabinol in an oral solution” from Schedule I into Schedule II.
Based on the FDA's evaluation of CAMU (currently accepted medical use), it concluded "that there is accepted safety for the use of marijuana under medical supervision for the treatment of anorexia related to a medical condition, nausea and vomiting (e.g., chemotherapy-induced), and pain."
Source: https://www.dea.gov/sites/default/files/2024-05/2016-17954-HHS.pdf
Learn More about Prodigy's Extraction Equipment
World Class cGMP and GMP Cannabis Extraction Technology

Learn More about FDA cGMP & EU GMP Compliance
The Professional's Choice for Cannabis Extraction Technology
Let's GET STARTED

Let's discuss your project
We look forward to transitioning your cannabis lab to FDA pharmaceutical compliant extraction standards!
Sign Up for the Latest News, Offers and Tips & Tricks of the Trade
Follow Us
More Articles & Pro Tips
Prodigy Processing Solutions Announces Strategic Partnership with inTEST Thermal Solutions
With increasing scrutiny on consumer safety of cannabis products coupled...
Read MoreProdigy Processing Solutions to be First Hydrocarbon Cannabis Extraction Equipment Approved for Use in New York City
With increasing scrutiny on consumer safety of cannabis products coupled...
Read MoreGMP Compliant Extraction Equipment
With increasing scrutiny on consumer safety of cannabis products coupled...
Read MoreChoosing the Right Cannabis Extraction Equipment
The equipment you choose for your cannabis extraction business is...
Read MoreCannabinoid API Manufacturing Benefits and Calculator
Delta-9 THC API Calculator | Input Your Own Values The...
Read MoreWhy Buying Used Cannabis Extraction Equipment Could Be a Costly Mistake
Applying a cost-benefit analysis to your capital expenditures In the...
Read MoreThe OCD Chef: Understanding GMP Compliance
Taking the mystery out of Good Manufacturing Practices Forward Note:...
Read MoreA Letter from Prodigy’s CEO to the DEA
Support of the DEA’s proposed rescheduling of marijuana from Schedule...
Read MoreThe Hidden Risks of Failing to Comply with GMP
What processors and dispensary owners need to know By Marc...
Read More










